The life sciences industry continues to evolve as organizations strive to improve the accuracy, speed, and compliance of their clinical documentation processes. One of the most critical components in this workflow is the Clinical Study Report, a detailed document submitted to regulatory authorities such as the Food and Drug Administration (FDA) at every phase of a new drug’s development.
Within each CSR, the Safety Evaluation Report plays a key role, summarizing crucial findings from complex clinical trial data. However, generating these summaries has traditionally been a manual, time intensive process, requiring clinical teams to extract insights from large, tabular datasets while ensuring alignment with regulatory writing standards.
To address these challenges, Tiger Analytics developed a Generative AI powered solution that automates the creation of Clinical Study Reports. The engagement demonstrated how AI can effectively streamline reporting workflows while maintaining compliance and accuracy.
The growing complexity of modern clinical programs means that documentation timelines are becoming increasingly compressed. Regulatory bodies expect greater transparency, deeper analytical rigor, and consistent writing quality across every submission. As a result, life sciences organizations are under mounting pressure to modernize how documentation is created, audited, and approved. Generative AI offers a unique opportunity to make this shift by combining data driven automation with human centered oversight, ensuring that reporting quality improves even as timelines shrink.
The Business Context: Manual Processes and Rising Complexity
Clinical teams typically go through CSR tables manually, extracting key information and drafting summaries in a predefined format. The process requires precision, domain expertise, and strict adherence to FDA reporting standards.
As drug portfolios expand and data volumes grow, manual CSR creation becomes increasingly difficult to scale. The challenge lies in balancing three critical demands:
- Ensuring data accuracy and correct interpretation of trial results.
- Maintaining writing consistency across authors and drug phases.
- Meeting tight regulatory submission timelines without compromising quality.
These factors collectively increase operational burden and raise the risk of reporting delays.
In many organizations, CSR creation cycles span several weeks or even months due to the number of reviews, manual iterations, and cross functional checkpoints involved. Medical writers often spend a significant portion of their time rewriting similar summaries for related drug programs or updated data cuts. As a result, highly skilled clinical experts are consumed with repetitive documentation work rather than dedicating time to deeper scientific analysis, risk mitigation, or strategic submission planning.
Streamlining CSR Creation Through AI
The need for automation in regulatory documentation has never been greater. By applying Generative AI, clinical and medical writing teams can reduce manual effort while ensuring precise, compliant reporting.
The opportunity was clear, to design a system capable of:
- Generating CSR Safety Evaluation Report summaries automatically.
- Presenting crucial discoveries from CSR tables in order of importance.
- Producing free text narratives from tabular data with proper data alignment.
- Scaling to additional CSR sections across multiple drug phases.
This approach allows teams to focus on analysis and validation rather than repetitive report preparation.
Generative AI not only accelerates document creation but also enables richer insights by identifying patterns that may not be immediately visible in raw tables. The technology can surface unusual event trends, distinguish meaningful signals from noise, and offer structured summaries that remain consistent across authors and therapeutic areas. When combined with domain specific guardrails, AI becomes a reliable partner in maintaining scientific integrity while improving efficiency.
Introducing Tiger’s Clinical Study Report Generator
The Clinical Study Report Generator is a GenAI powered solution that automates extraction, summarization, and report assembly for clinical trial data. It generates concise, regulation compliant Safety Evaluation sections directly from complex CSR tables, streamlining the creation of FDA ready documents.
Powered by Databricks: Scalable Data and AI Foundation
The Clinical Study Report Generator was built on the Databricks Data Intelligence Platform, which served as the unified foundation for managing large scale clinical data, model fine tuning, and automated report generation. Using Databricks, the solution efficiently ingested and processed structured and unstructured data including PDFs, HTMLs, and CSR in text tables while maintaining consistency and lineage through Delta Lake. Data engineers leveraged Databricks notebooks to automate the extraction of headers, table values, and section specific logic, enabling standardized and high quality inputs for downstream AI processing.
Within this environment, Large Language Models (LLMs) were fine tuned using techniques such as Parameter Efficient Fine Tuning (PEFT) and Low Rank Adaptation (LoRA) to align outputs with CSR specific sections and regulatory writing patterns. The Databricks ecosystem supported continuous validation through MLflow, ensuring transparent tracking of model performance and prompt iterations. This integrated setup enabled the automated generation of FDA compliant Safety Evaluation Reports, transforming complex clinical data into structured, regulation ready narratives while ensuring scalability, accuracy, and compliance across drug development phases.
A key differentiator of this platform based approach is its ability to dynamically incorporate new data cuts without requiring manual redesign of pipelines or templates. As trials progress and interim analyses evolve, the system can automatically refresh summaries and flag changes across versions, allowing teams to maintain complete control over documentation traceability. This ensures that submissions remain audit ready at every stage.
Technical Architecture
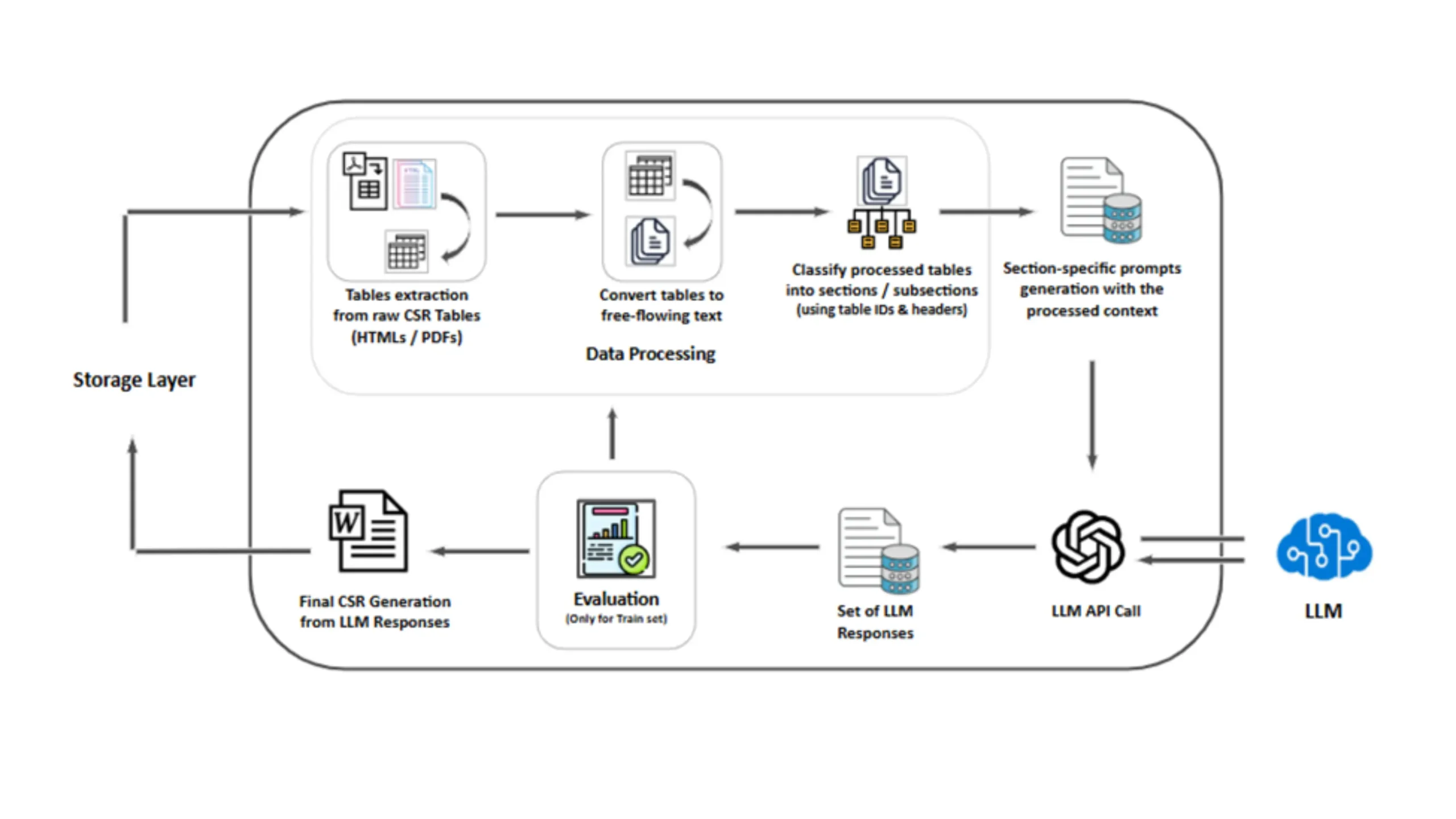
Solution Components
- Data Parsing: Extracts structured tabular data and text from CSR documents.
- Summarization Module: Uses large language models to generate lean summaries for safety sections.
- Report Builder Engine: Assembles section level summaries into fully formatted, FDA aligned CSR documents.
- Validation Assistant: Enables human in loop review and prompt tuning to ensure precision and accuracy.
Together, these modules deliver end to end CSR automation, turning raw clinical data into structured, compliant narratives.
The modular nature of the solution ensures that organizations can adopt it incrementally. Teams may begin with AI assisted summarization before expanding to full report generation, enabling a smooth and controlled adoption curve. This approach respects existing medical writing workflows while introducing automation where it adds immediate value.
Key Features and Differentiators
- End to End CSR Automation: Processes raw trial data to create complete Safety Evaluation sections.
- LLM Powered Summarization: Generates concise, structured, and regulation compliant text.
- Multi Level Data Flattening and Tagging: Maintains data hierarchy and severity classifications.
- Human in Loop Feedback Integration: Supports iterative accuracy improvement through validation cycles.
- Scalable Architecture: Extendable across multiple drug phases and CSR sections.
- FDA Alignment: Ensures outputs follow strict regulatory documentation standards.
Additionally, the solution incorporates robust data quality checks that automatically flag missing values, contradictory entries, or outlier events. These checks help reduce back and forth between biostatistics, clinical, and writing teams, significantly improving the end to end turnaround time for each CSR milestone.
Sample Output

Business Value Delivered
The Clinical Study Report Generator delivers measurable benefits across clinical and regulatory teams:
- Effort Reduction: Cuts manual writing across CSR cycles.
- Faster Submissions: Automates FDA ready summaries, reducing turnaround times.
- Improved Consistency: Minimizes variation across authors and trial phases.
- Higher Analyst Productivity: Enables teams to focus on insights and validation over formatting.
- Scalable Application: Adaptable to additional CSR sections and new drug programs.
This transformation enhances operational efficiency while ensuring adherence to regulatory standards.
Organizations adopting this solution have observed improved version control, reduced dependency on external medical writers, and greater confidence during internal and external audits. By embedding compliance rules within the AI workflow, teams ensure that every generated document follows the same rigorous structure, reducing the likelihood of submission rework or follow up queries from authorities.
The Future of Clinical Documentation
As life sciences organizations face increasing regulatory complexity, AI driven documentation solutions like the Clinical Study Report Generator are becoming essential.
By combining domain expertise with advanced language models, Tiger Analytics enables clinical teams to move from manual report generation to automated, insight driven workflows, ensuring precision, compliance, and scalability for the next generation of regulatory reporting.
In the years ahead, AI powered documentation will likely expand beyond CSRs to include protocols, statistical analysis plans, investigator brochures, and even patient narratives. These advancements will create a unified and intelligent ecosystem for regulatory documentation that is traceable, standardized, and deeply integrated with clinical data workflows. The Clinical Study Report Generator marks a significant step in this transformation, enabling organizations to build a future ready documentation strategy that evolves alongside scientific innovation.

